Proc. Natl. Acad. Sci. USA 109, 18713-18718 (2012)
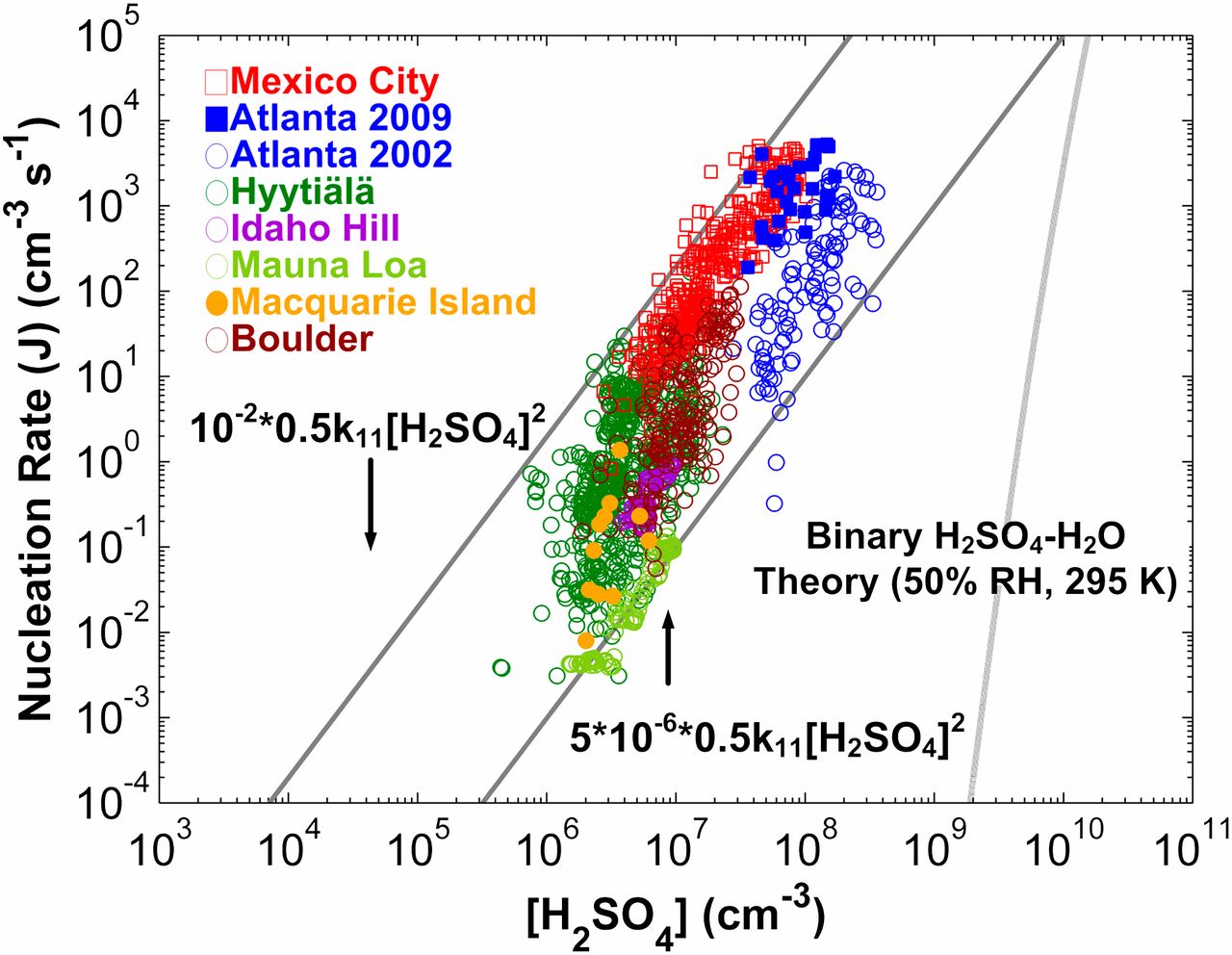
Climate models show that particles formed by nucleation can affect cloud cover and, therefore, the earth's radiation budget. Measurements worldwide show that nucleation rates in the atmospheric boundary layer are positively correlated with concentrations of sulfuric acid vapor. However, current nucleation theories do not correctly predict either the observed nucleation rates or their functional dependence on sulfuric acid concentrations. This paper develops an alternative approach for modeling nucleation rates, based on a sequence of acid–base reactions. The model uses empirical estimates of sulfuric acid evaporation rates obtained from new measurements of neutral molecular clusters. The model predicts that nucleation rates equal the sulfuric acid vapor collision rate times a prefactor that is less than unity and that depends on the concentrations of basic gaseous compounds and preexisting particles. Predicted nucleation rates and their dependence on sulfuric acid vapor concentrations are in reasonable agreement with measurements from Mexico City and Atlanta.