J. Chem. Eng. Data 59, 3301-3306 (2014)
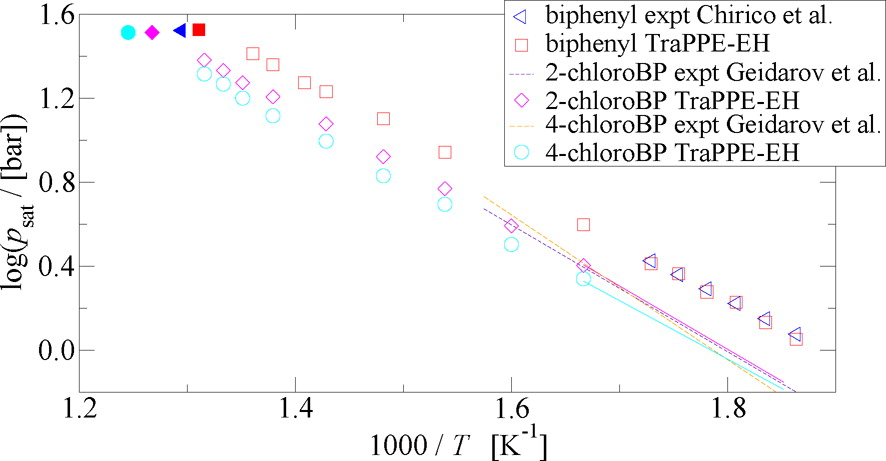
Gibbs ensemble Monte Carlo simulations using the explicit-hydrogen version of the transferable potentials for phase equilibria (TraPPE–EH) force field were carried out to predict the vapor–liquid coexistence and critical properties of biphenyl, monochlorinated biphenyls, and of 16 polychlorinated biphenyls. The predictions are in very good agreement with the limited experimental data. Transferring the TraPPE–EH Lennard-Jones parameters from benzene to construct biphenyl yields predicted critical properties and normal boiling temperature with an average deviation of less than 1 %. The saturated vapor pressures for biphenyl, 2-chorobiphenyl, and 4-chlorobiphenyl fall within 10 % of the experimental data. Overall, the critical temperatures increase nearly linearly with the number of chlorine substituents and are correlated with the dipole moment for the monochlorinated isomers. In contrast, 4,4′-dichlorobiphenyl, the most elongated compound, exhibits the highest critical temperature among the disubstituted biphenyls.