Angew. Chem. Intl. Ed. 55, 5938-5942 (2016)
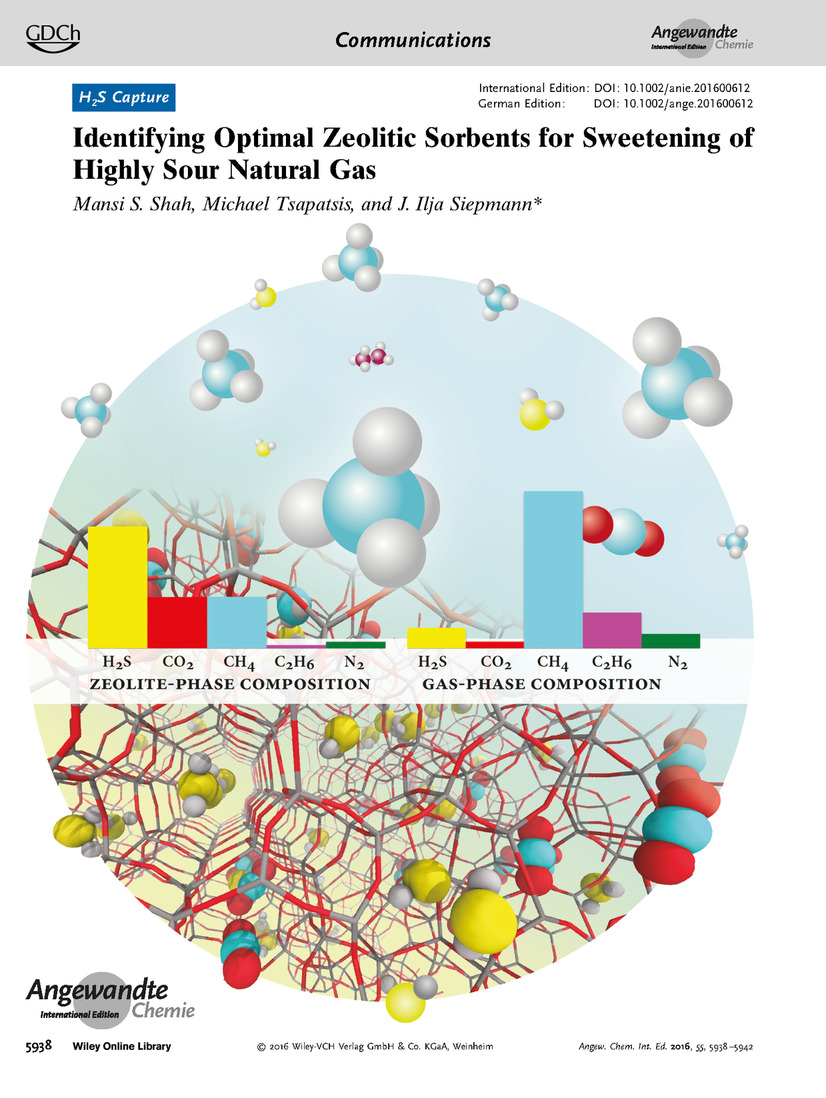
Raw natural gas is a complex mixture comprising methane, ethane, other hydrocarbons, hydrogen sulfide, carbon dioxide, nitrogen, and water. For sour gas fields, selective and energy‐efficient removal of H2S is one of the crucial challenges facing the natural‐gas industry. Separation using nanoporous materials, such as zeolites, can be an alternative to energy‐intensive amine‐based absorption processes. Herein, the adsorption of binary H2S/CH4 and H2S/C2H6 mixtures in the all‐silica forms of 386 zeolitic frameworks is investigated using Monte Carlo simulations. Adsorption of a five‐component mixture is utilized to evaluate the performance of the 16 most promising materials under close‐to‐real conditions. It is found that depending on the fractions of CH4, C2H6, and CO2, different sorbents allow for optimal H2S removal and hydrocarbon recovery.