Mol. Phys. 116, 3283–3291 (2018)
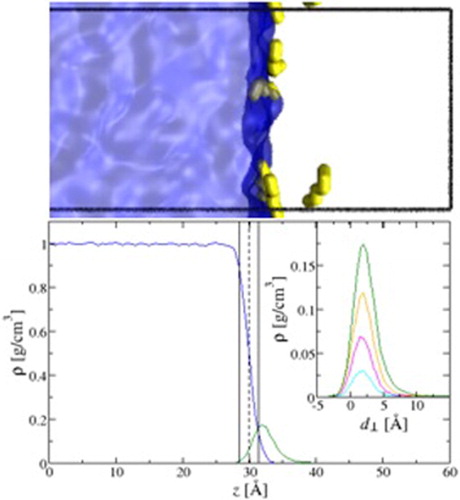
Knowledge about the interfacial properties of water/oil mixtures is important for the petrochemical industry and for understanding detergency and hydrophobic effects. Here, we probe the liquid/vapour interface of water/n-hexane mixtures using configurational-bias Monte Carlo simulations in the NWNHVHpHT osmotic Gibbs ensemble. We study the effect of n-hexane at several partial pressures ranging from 25% to 95% of its saturated vapour pressure and observe that the surface tension decreases with increasing n-hexane pressure. Additionally, we analyse the simulation trajectories to provide molecular-level insights on the spatial distribution of n-hexane and the structure of the interface. The n-hexane molecules strongly adsorb from the vapour phase onto the liquid interface with a preferentially parallel orientation with respect to the interface. The surface excess, from the Gibbs adsorption isotherm equation, is calculated and used to systematically define the domain of adsorbed n-hexane. Integrating over this gives the free energy of adsorption of n-hexane, which is highly favourable, varying from -9.56 +/- .03 to -10.40 +/- .02 kJ/mol as the partial pressure of n-hexane is increased. The enrichment of n-hexane molecules on the interface yields a positive deviation from Henry's law at higher partial pressures, providing evidence for favourable adsorbate-adsorbate interactions.