J. Phys. Chem. C 117, 24375-24387 (2013)
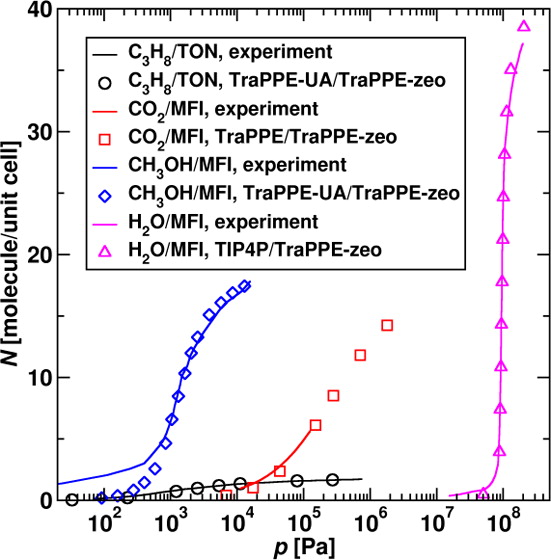
The transferable potentials for phase equilibria (TraPPE) force field is extended to all-silica zeolites. This novel force field is parametrized to match the experimental adsorption isotherms of n-heptane, propane, carbon dioxide, and ethanol with the Lennard-Jones parameters for sorbate–framework interactions determined in a consistent manner using the Lorentz–Berthelot combining rules as for other parts of the TraPPE force field. The TraPPE-zeo force field allows for accurate predictions for both adsorption and diffusion of alkanes, alcohols, carbon dioxide, and water over a wide range of pressures and temperatures. In order to achieve transferability to a wider range of molecule types, ranging from nonpolar to dipolar and hydrogen-bonding compounds, Lennard-Jones interaction sites and partial charges are placed at both the oxygen and the silicon atoms of the zeolite lattice, which allows for a better balance of dispersive and first-order electrostatic interactions than is achievable with the Lennard-Jones potential used only for the oxygen atoms. The use of the Lorentz–Berthelot combining rules for unlike interactions makes the TraPPE-zeo force field applicable to any sorbate as long as the relevant TraPPE sorbate–sorbate parameters are available. The TraPPE-zeo force field allows for greatly improved predictive power compared to force fields that explicitly tabulate the individual cross-interaction parameters.