J. Phys. Chem. C 118, 19723-19732 (2014)
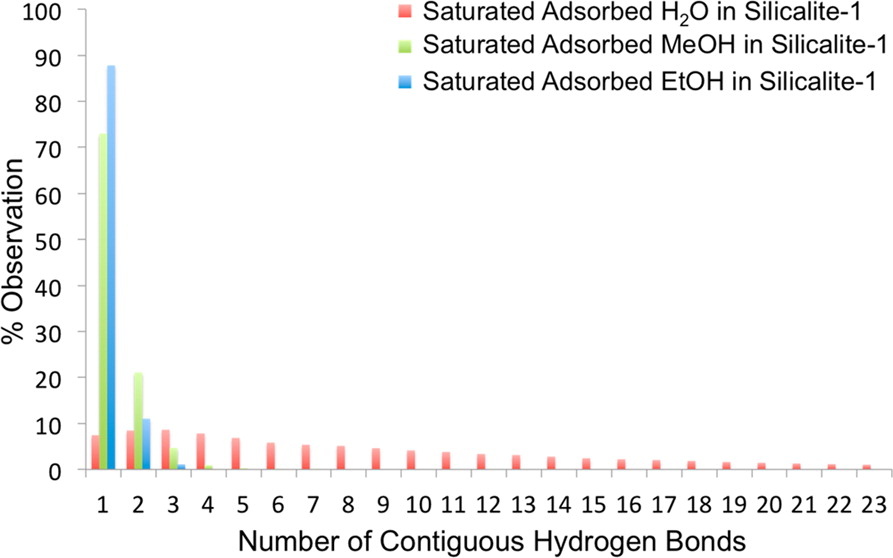
Essential topological indices of the hydrogen-bond networks of water, methanol, ethanol, and their binary mixtures adsorbed in microporous silicalite-1 (a hydrophobic zeolite with potential application for biofuel processing) are analyzed and compared to their bulk liquid counterparts. These include the geodesic distribution (the shortest H-bond pathways between molecular vertices), the average length, the geodesic index, the orientation and distance of the adsorbate to the interior of the zeolite, and the sorbate–sorbate and sorbate–sorbent distributions of H-bonds. In combination, they describe how the H-bond networks are altered when going from the bulk to the confined silicalite-1 environment. The speciation of the adsorbed compounds is quantified in terms of their network connectivity, revealing that pure water has a high probability of forming long, contiguous H-bonded chains in silicalite-1 at high loading, while alcohols form small dimeric/trimeric clusters. The extent to which the H-bond network of binary water–alcohol systems is altered relative to either unary system is quantified, demonstrating an enhanced interconnectivity that is reflected in the tendency of individual H2O molecules to become co-adsorbed with alcohol clusters in the zeolite framework. Selectivity for the alcohol over water diminishes with increasing alcohol loading as the H-bonded clusters serve as favorable adsorption sites for H2O.