J. Chem. Phys. 142, art. no. 044902/13 pages (2015)
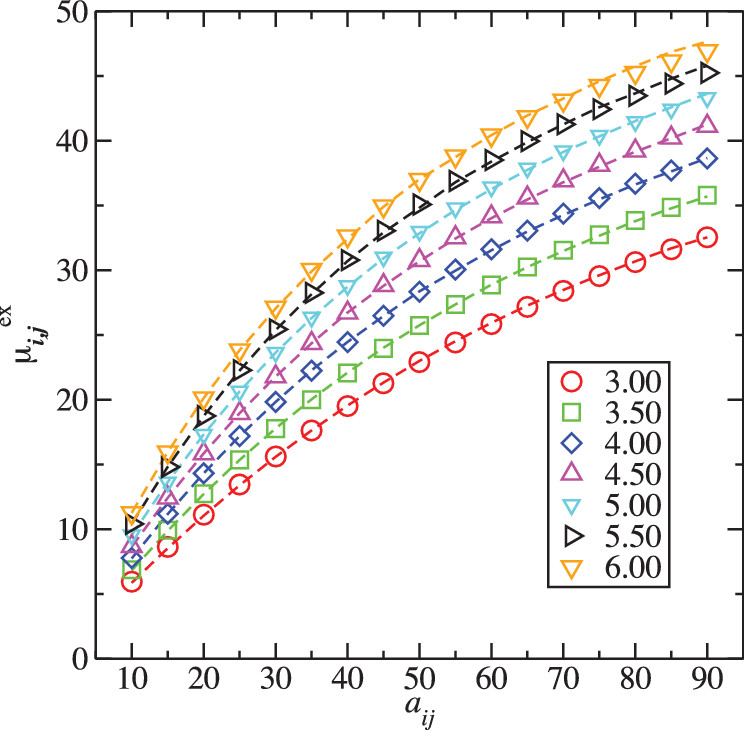
Three developments are presented that significantly expand the applicability of dissipative particle dynamics (DPD) simulations for symmetric and non-symmetric mixtures, where the former contain particles with equal repulsive parameter for self-interactions but a different repulsive parameter for cross-interactions, and the latter contain particles with different repulsive parameters also for the self-interactions. Monte Carlo and molecular dynamics simulations for unary phases covering a wide range of repulsive parameters and of densities for single-bead DPD particles point to deficiencies of the Groot and Warren equation of state (GW-EOS) [J. Chem. Phys. 107, 4423 (1997)]. A revised version, called rGW-EOS, is proposed here that is significantly more accurate over a wider range of parameters/densities. The second development is the generalization of the relationship between the Flory-Huggins χ parameter and the repulsive cross-interaction parameter when the two particles involved have different molecular volumes. The third aspect is an investigation of Gibbs ensemble Monte Carlo simulation protocols, which demonstrates the importance of volume fluctuations and excess volumes of mixing even for equimolar symmetric mixtures of DPD particles. As an illustrative example, the novel DPD methodology is applied to the prediction of the liquid–liquid equilibria for acetic anhydride/(n-hexane or n-octane) binary mixtures.