[27] Intermolecular potentials for branched alkanes and the vapour-liquid phase equilibria of n-heptane, 2-methylhexane, and 3-ethylpentane
Mol. Phys. 90, 687-693 (1997)
Publication Abstract
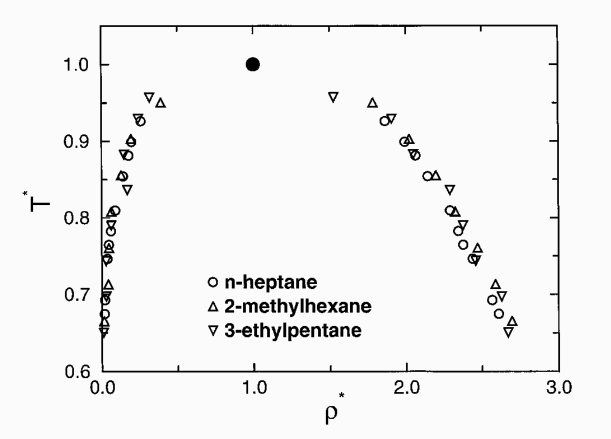
Configurational bias Monte Carlo calculations in the Gibbs ensemble have been used to perform direct simulations of the vapour-liquid phase equilibria of three heptane isomers: n-heptane, 2-methylhexane, and 3-ethylpentane. The simulations were carried out using isotropic united-atom representations of the Lennard-Jones type for the CH3, CH2 and CH groups. The aim of these calculations is to extend our force field, previously derived for linear alkanes, to branched alkanes by fitting new interaction parameters for methyl and ethyl branches.