J. Phys. Chem. C 2021, 125, 9, 5380–5385 (2021)
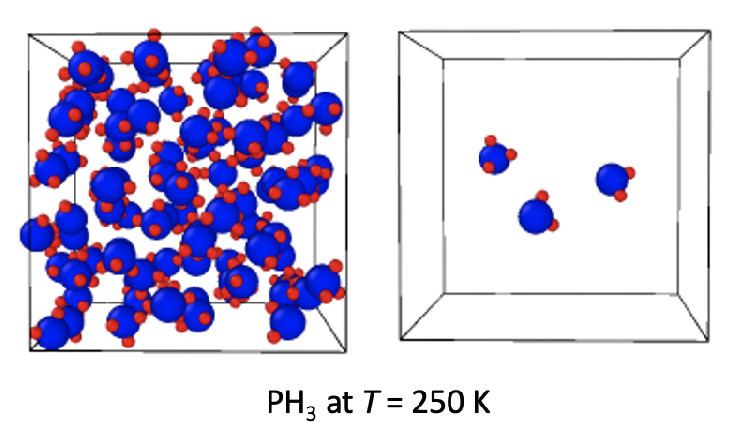
Because of their toxicity, flammability, and chemical instability, the vapor–liquid equilibria of the group V hydrides, phosphine, arsine, and stibine are underexplored. Gibbs ensemble Monte Carlo simulations were performed to obtain the vapor–liquid coexistence curves (VLCCs) for PH3, AsH3, and SbH3 by using Kohn–Sham density functional theory with two popular generalized gradient approximation functionals to describe the inter- and intramolecular interactions. In conjunction with the third-generation dispersion correction of Grimme, Goedecker–Teter–Hutter pseudopotentials, and double-ζ valence polarization basis sets, the Perdew–Burke–Ernzerhof (PBE) and Becke–Lee–Yang–Parr (BLYP) exchange-correlation functionals yield critical temperatures for PH3 of 342 ± 8 and 289 ± 12 K, respectively, that overpredict/underpredict the experimental value by about 20 and 35 K. The high computational cost of first-principles simulations necessitates relatively small system sizes and short simulations that can lead to problems near the critical point. No significant system size effects were observed for the VLCC and liquid structure of PH3. For AsH3 and SbH3, the predicted critical temperatures with the PBE exchange-correlation functional are close to 450 K, a value about 20% above the available literature value for AsH3.