Ind. Eng. Chem. Res. 60, 739–752 (2021)
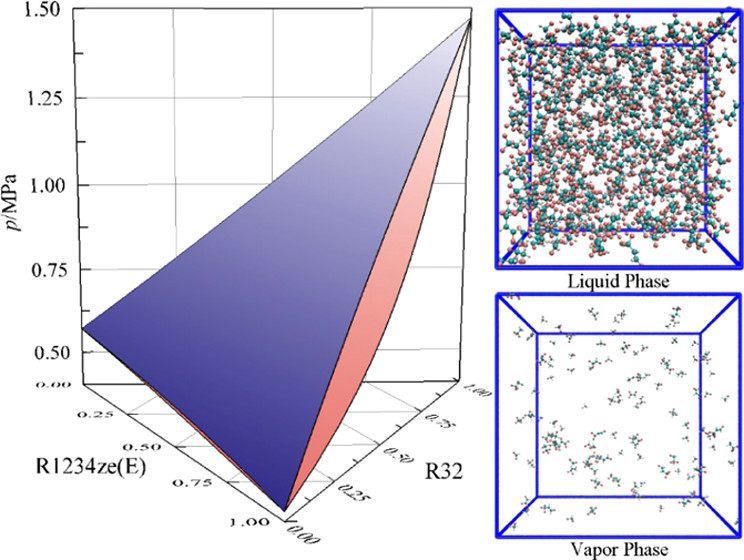
The combination of difluoromethane (R32), 1,1,1,2-tetrafluoroethane (R134a), and trans-1,3,3,3-tetrafluoro-1-propene (R1234ze(E)) has been recently proposed as a potential substitute (R456A) for hydrofluorocarbon working fluids. For the design and process simulation of refrigeration systems using refrigerant mixtures, precise knowledge of their thermophysical properties, especially vapor–liquid equilibrium (VLE), is crucial. To extend the experimental temperature range, a liquid-recirculation analytical apparatus, classified as AnTLcirCapValVis, was redesigned and VLE data for the binary mixtures of R32 + R134a, R32 + R1234ze(E), R134a + R1234ze(E), and the ternary system of R32 + R134a + R1234ze(E) were measured over the temperature range from 263.15 to 323.15 K. The standard uncertainties of the temperature, pressure, and the mole fractions of liquid and vapor phases are estimated to be within 10 mK, 0.5 kPa, and 0.005, respectively. The Peng–Robinson–Stryjek–Vera–Version-2 (PRSV2) equation of state combined with the Wong–Sandler (WS) mixing rule and the nonrandom two-liquid activity coefficient model (NRTL) was used to fit the mixing parameters of the binary data from this work and prior studies and to predict the ternary VLE properties. In addition, Gibbs ensemble Monte Carlo simulations with an all-atom force field were carried out to determine VLE properties and to characterize the microscopic structure of these mixtures. Good agreement is found between experiments, correlations, and simulations, which attests to the predictive capabilities of the PRSV2 + WS + NRTL model and molecular simulations.