J. Phys. Chem. B 126, 3940–3949 (2022)
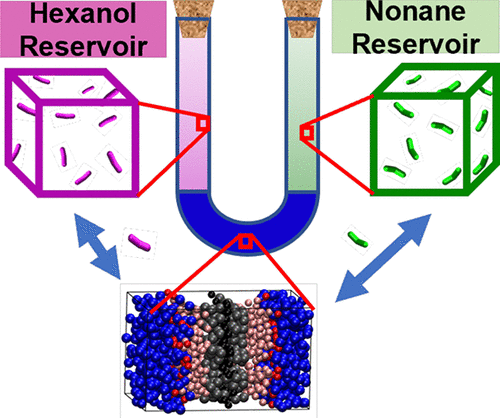
Adsorption of n-nonane/1-hexanol (C9/C6OH) mixtures into the lamellar phase formed by a 50/50 w/w triethylene glycol mono-n-decyl ether (C10E3)/water system was studied using configurational-bias Monte Carlo simulations in the osmotic Gibbs ensemble. The interactions were described by the Shinoda–Devane–Klein coarse-grained force field. Prior simulations probing single-component adsorption indicated that C9 molecules preferentially load near the center of the bilayer, increasing the bilayer thickness, whereas C6OH molecules are more likely to be found near the interface of the polar and nonpolar moieties, swelling the bilayer in the lateral dimension. Here, we extend this work to binary C9/C6OH adsorption to probe whether the difference in the spatial preferences may lead to a synergistic effect and enhanced loadings for the mixture. Comparing loading trends and the thermodynamics of binary adsorption to unary adsorption reveals that C9–C9 interactions lead to the largest enhancement, whereas C9–C6OH and C6OH–C6OH interactions are less favorable for this bilayer system. Ideal adsorbed solution theory yields satisfactory predictions of the binary loading.