Anal. Chem. 79, 6551-6558 (2007)
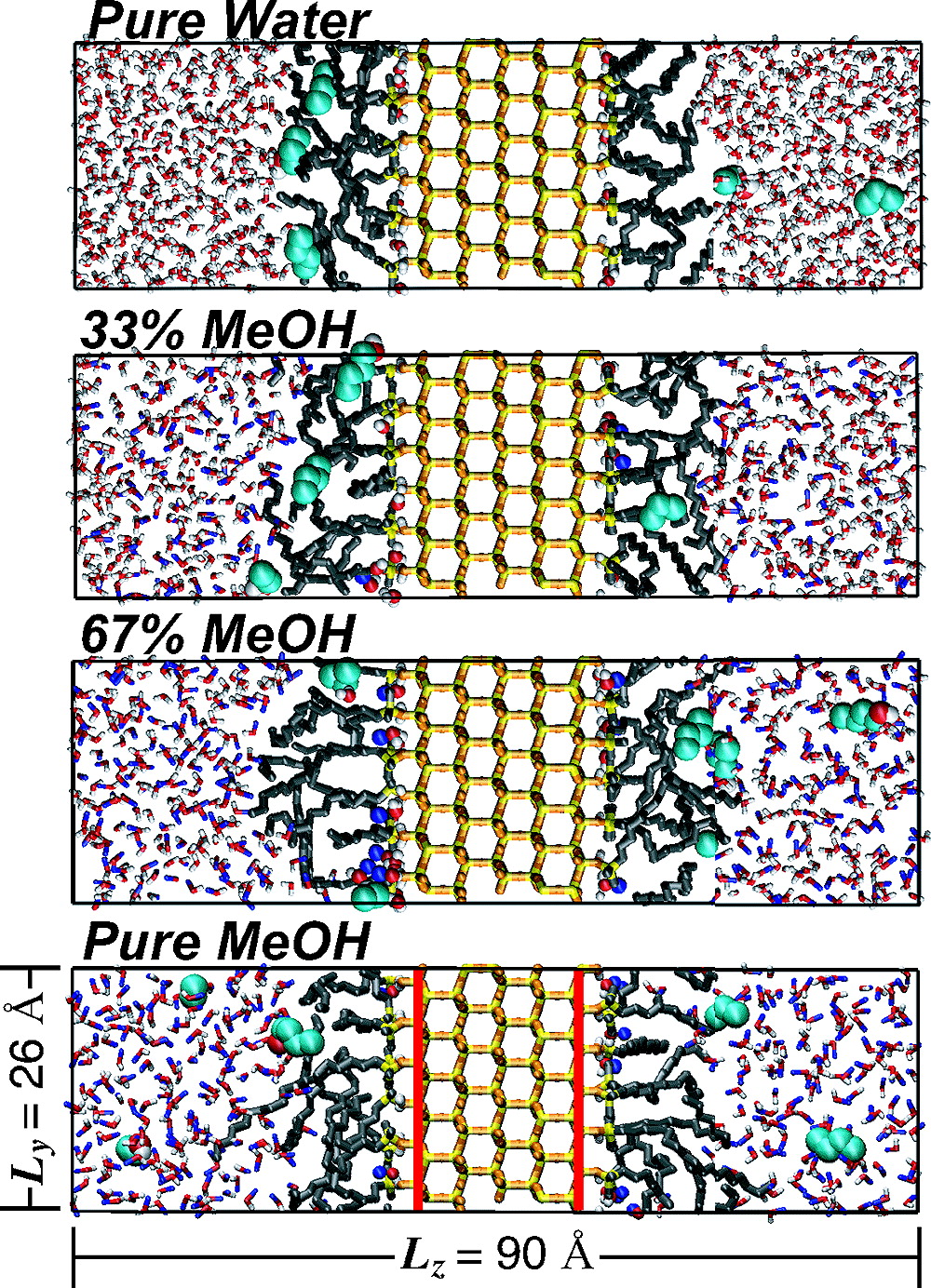
A detailed, molecular-level understanding of the retention mechanism in reversed-phase liquid chromatography (RPLC) has eluded analytical chemists for decades. Through validated, particle-based Monte Carlo simulations of a model RPLC system consisting of dimethyloctadecylsilanes at a coverage of 2.9 μmol/m2 on an explicit silica substrate with unprotected residual silanols in contact with a water/methanol mobile phase, we show that the molecular-level retention processes for nonpolar and polar analytes, such as alkanes and alcohols, are much more complex than what has been previously deduced from thermodynamic and theoretical arguments. In contrast to some previous assumptions, the simulations indicate that both partitioning and adsorption play a key role in the separation process and that the stationary phase in RPLC behaves substantially different from a bulk hydrocarbon phase. The retention of nonpolar methylene segments is dominated by lipophilic interactions with the retentive phase, while solvophilic interactions are more important for the retention of the polar hydroxyl group.