J. Phys. Chem. B 111, 10790-10799 (2007)
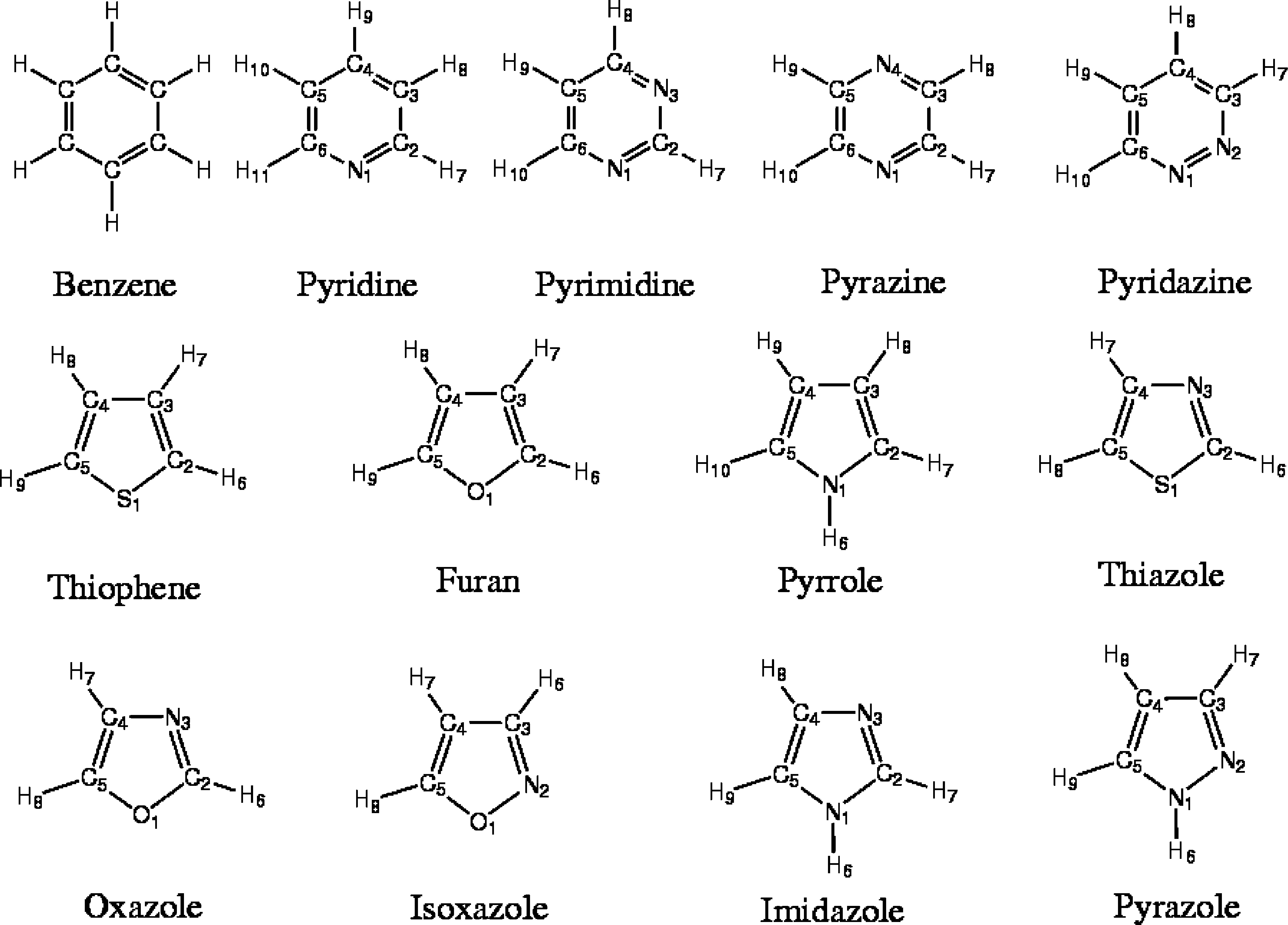
The explicit hydrogen version of the transferable potentials for phase equilibria (TraPPE-EH) force field is extended to benzene, pyridine, pyrimidine, pyrazine, pyridazine, thiophene, furan, pyrrole, thiazole, oxazole, isoxazole, imidazole, and pyrazole. While the Lennard-Jones parameters for carbon, hydrogen (two types), nitrogen (two types), oxygen, and sulfur are transferable for all 13 compounds, the partial charges are specific for each compound. The benzene dimer energies for sandwich, T-shape, and parallel-displaced configurations obtained for the TraPPE-EH force field compare favorably with high-level electronic structure calculations. Gibbs ensemble Monte Carlo simulations were carried out to compute the single-component vapor−liquid equilibria for benzene, pyridine, three diazenes, and eight five-membered heterocycles. The agreement with experimental data is excellent with the liquid densities and vapor pressures reproduced within 1 and 5%, respectively. The critical temperatures and normal boiling points are predicted with mean deviations of 0.8 and 1.6%, respectively.