J. Phys. Chem. C 111, 15634-15641 (2007)
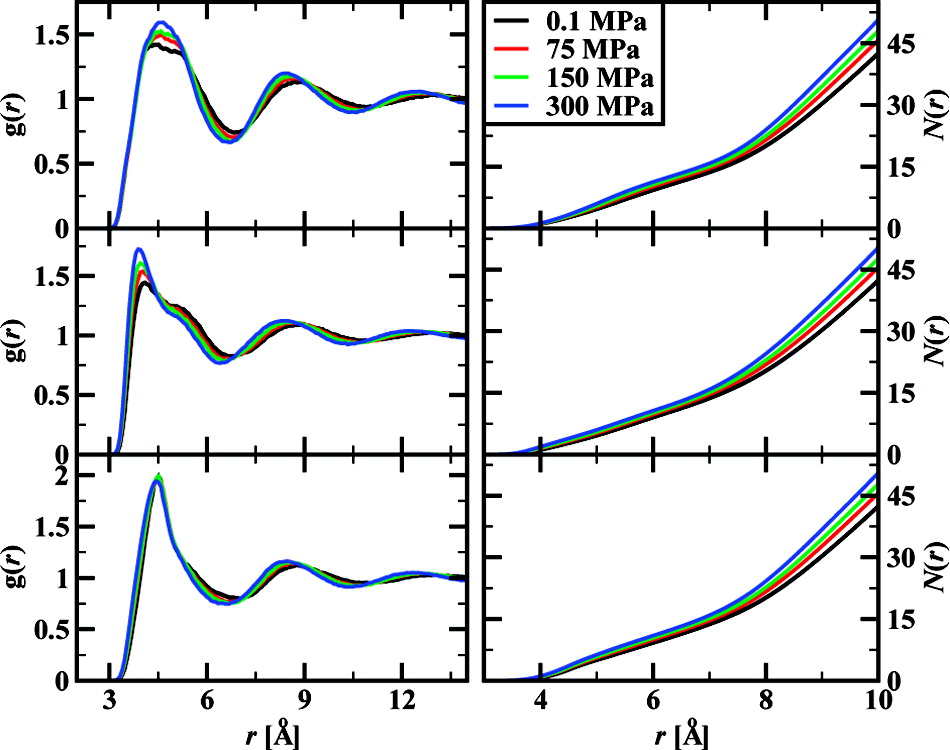
Monte Carlo simulations were carried out to compute the Hildebrand solubility parameter and the internal pressure for n-hexane, benzene, and ethanol at a temperature of 303.15 K and for external pressures ranging from 0.1 to 300 MPa. In addition, the internal energy, molar volume, enthalpy of the liquid phase, and free energy, enthalpy, and entropy of solvation were calculated as a function of external pressure. For all three molecules, the solubility parameter is found to increase monotonically with increasing external pressure, while the internal pressure exhibits a maximum. The magnitude of the solvation free energy decreases monotonically as external pressure is increased and both enthalpic and entropic terms contribute to this decrease in solubility. Analysis of radial distribution functions indicates small structural changes in the liquid phase and differences between nonpolar and hydrogen-bonding fluids in response to external pressure changes.