J. Phys. Chem. C 112, 210-218 (2008)
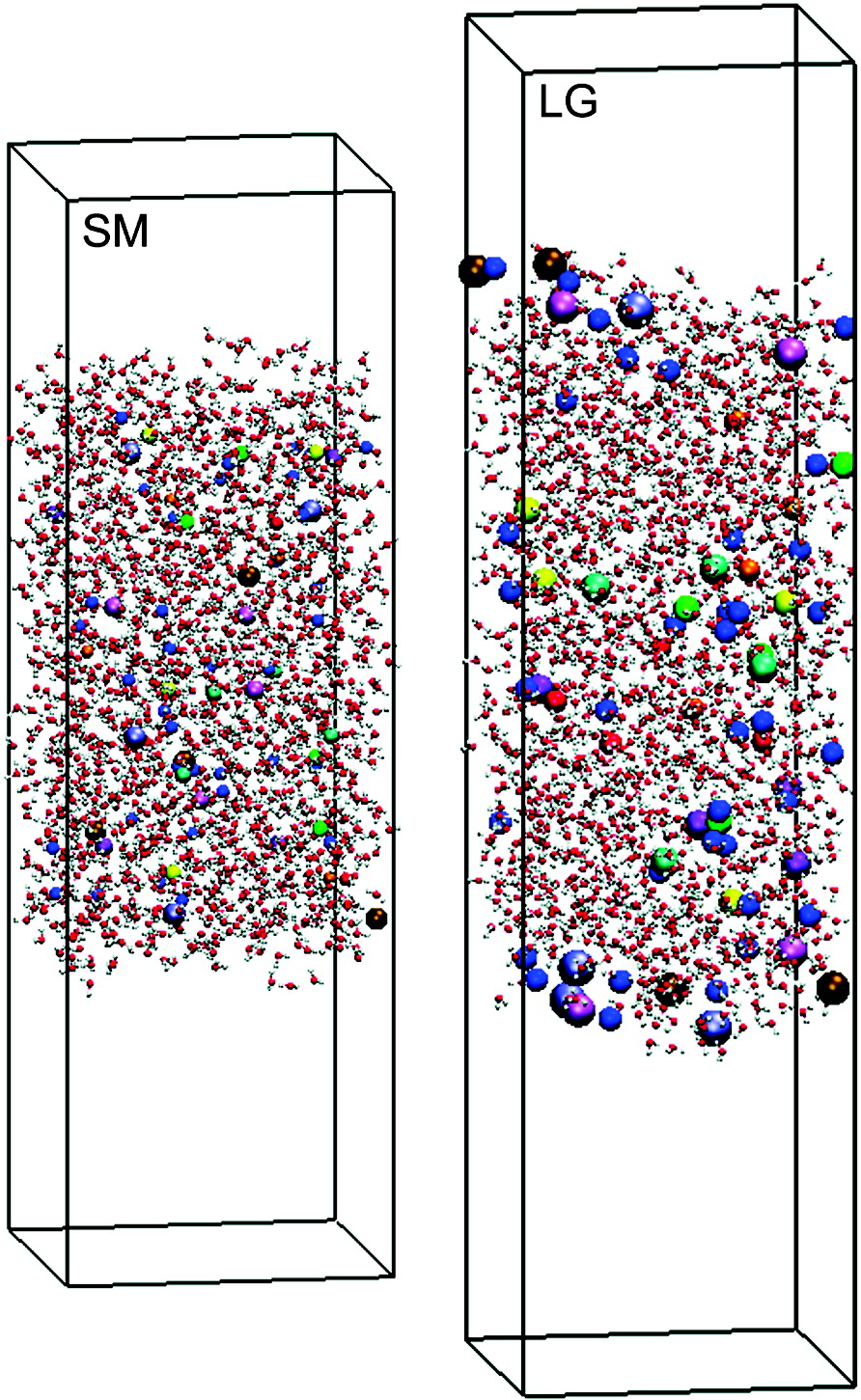
The presence of certain anions at the aqueous liquid−vapor interface is often attributed to polarization effects. This work shows that the size of the ion also plays an important role in driving monovalent inorganic ions to the surface. Configurational-bias Monte Carlo simulations in the Gibbs ensemble were performed to investigate the liquid−vapor interface for neat water and ionic solutions at 298 K. The total ion concentration is about 1.2 M, but the solutions contained a mixture of anions of varying size all having the same fixed charge and Lennard-Jones well depth (i.e., the same polarizability). Results show that as the size of the anion increases so does the propensity for interfacial solvation. The largest anions are consistently found closer to the interface than smaller anions. We also find that large anions, which may prefer a surface location on their own, can be excluded from the interfacial region by inclusion of even larger anions in a mixture.