J. Chem. Theor. Comp. 4, 136-144 (2008)
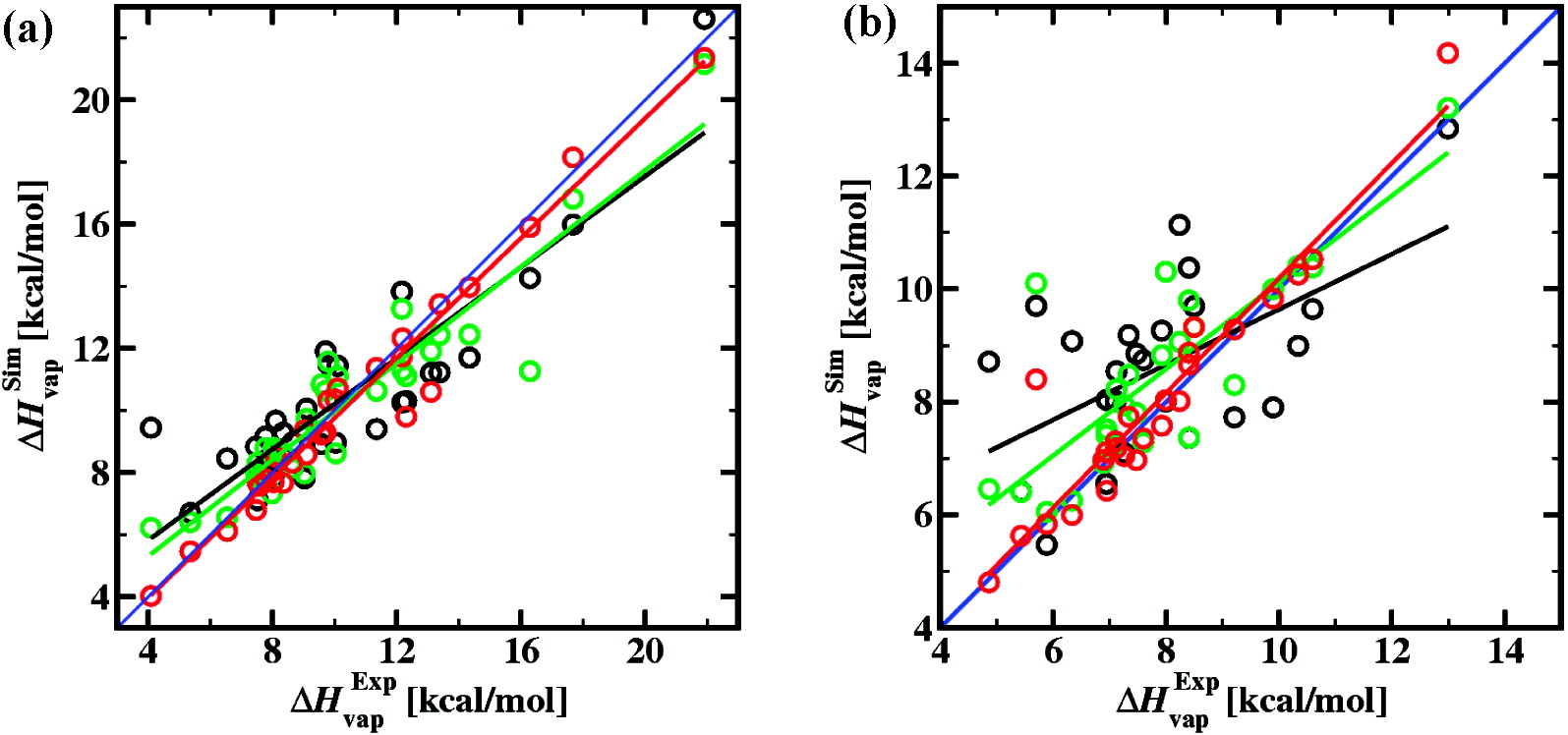
Configurational-bias Monte Carlo simulations in the isothermal−isobaric and Gibbs ensembles using the transferable potentials for phase equilibria (TraPPE) force field were carried out to compute the liquid densities, the Hildebrand solubility parameters, and the heats of vaporization for a set of 32 organic molecules with different functional groups at a temperature of 298.15 K. In addition, the heats of vaporization were determined at the normal boiling points of these compounds. Comparison to experimental data demonstrates that the TraPPE force field is significantly more accurate than predictions obtained from molecular dynamics simulations with the Dreiding force field [Belmares et al. J. Comput. Chem. 2004, 25, 1814] and an equation of state approach [Stefanis et al. Fluid Phase Equil. 2006, 240, 144]. For the TraPPE force field, the mean unsigned percent errors for liquid density, the Hildebrand solubility parameter, and the heat of vaporization at 298.15 K are 1.3, 3.3, and 4.5%, respectively.