Anal. Chem. 80, 6214-6221 (2008)
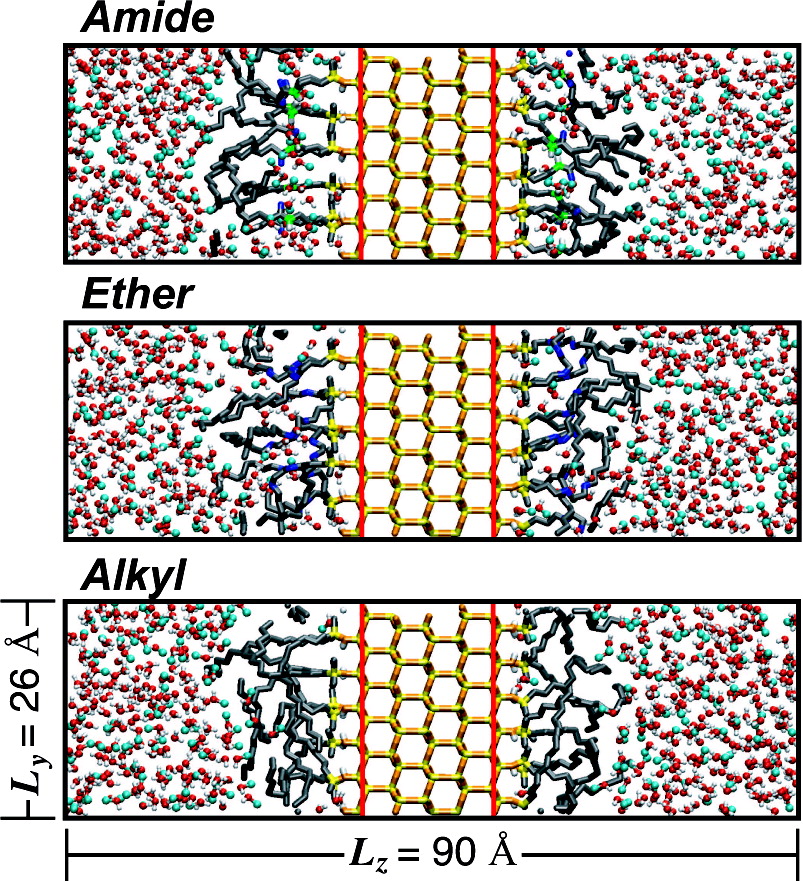
Stationary phases with embedded polar groups possess several advantages over conventional alkylsilane phases, such as reduced peak tailing, enhanced selectivity for specific functional groups, and the ability to use a highly aqueous mobile phase. To gain a deeper understanding of the retentive properties of these reversed-phase packings, molecular simulations were carried out for three different stationary phases in contact with mobile phases of various water/methanol ratios. Two polar-embedded phases were modeled, namely, amide and ether containing, and compared to a conventional octadecylsilane phase. The simulations show that, due to specific hydrogen bond interactions, the polar-embedded phases take up significantly more solvent and are more ordered than their alkyl counterparts. Alkane and alcohol probe solutes indicate that the polar-embedded phases are less retentive than alkyl phases for nonpolar species, whereas polar species are more retained by them due to hydrogen bonding with the embedded groups and the increased amount of solvent within the stationary phase. This leads to a significant reduction of the free-energy barrier for the transfer of polar species from the mobile phase to residual silanols, and this reduced barrier provides a possible explanation for reduced peak tailing.