J. Phys. Chem. B 112, 13005-13014 (2008)
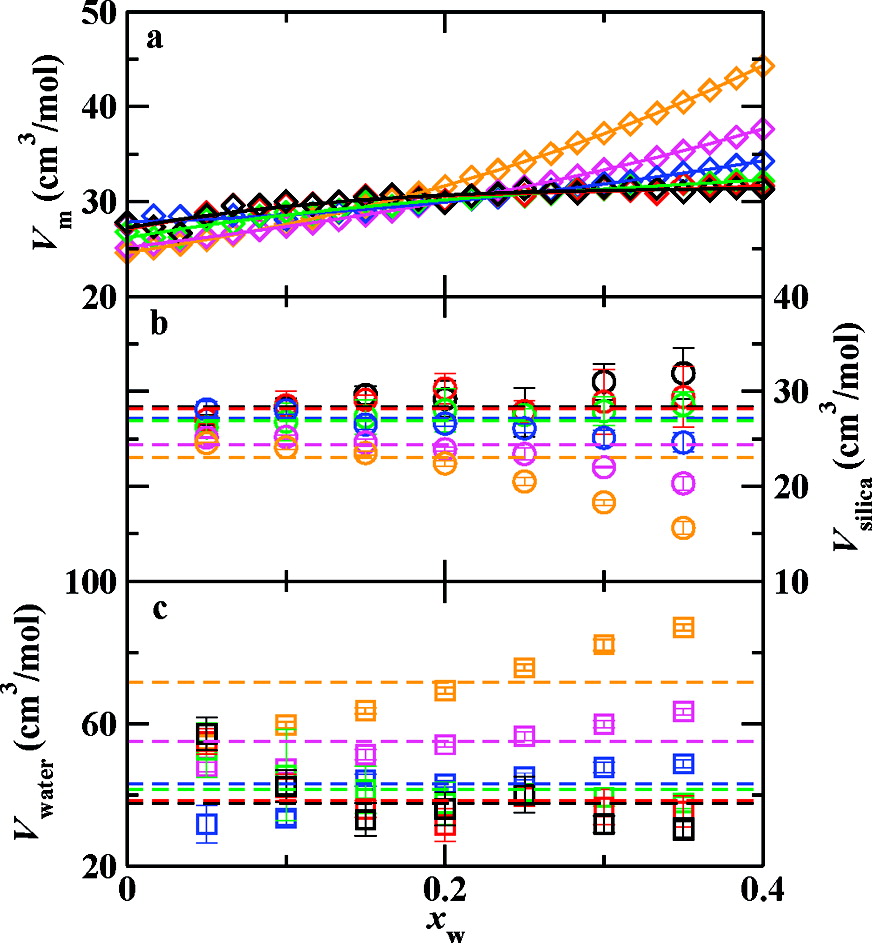
Monte Carlo simulations were used to investigate the phase behavior of hydrated liquid silica as a function of temperature and overall water mole fraction, xw. Simulations using the Feuston−Garofalini potential were performed in the isobaric−isothermal ensemble at p = 1 GPa for 15 temperatures (2000 ≤ T ≤ 9000 K) and 25 compositions (0.0 ≤ xw ≤ 0.4). The unusual volume minimum exhibited by tetrahedrally coordinated liquid silica is found to persist up to xw ≈ 0.267, although the temperature of the volume minimum decreases with increasing water content. Structural properties of the pure and hydrated systems are compared and the addition of water to liquid silica disrupts the silica network more dramatically than temperature alone. The simulations yield very low concentrations of molecular water, e.g. only about 1.2% of the oxygen atoms are bound to exactly two hydrogen atoms at xw = 0.4 and T = 3000 K.