[120] Importance of the Number of Acid Molecules and the Strength of the Base for Double-Ion Formation in (H2SO4)m·Base·(H2O)6 Clusters
J. Am. Chem. Soc. 130, 14144-14147 (2008)
Publication Abstract
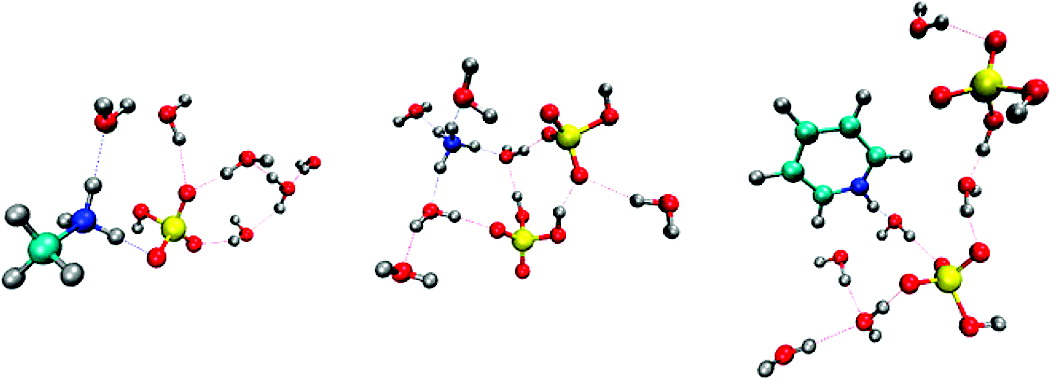
Sulfuric acid and water clusters are important for new particle formation in the atmosphere. Recent experimental studies demonstrate that critical clusters in diverse atmospheric environments contain two acid molecules and may also include additional N-containing molecules (i.e., a base). We use first-principles molecular dynamics simulations to show that the presence of two sulfuric acid molecules in (H2SO4)m·base·(H2O)6 clusters is always sufficient to form a double ion, whereas a single acid molecule, even in the presence of a base, is not.