J. Phys. Chem. A 113, 2053-2059 (2009)
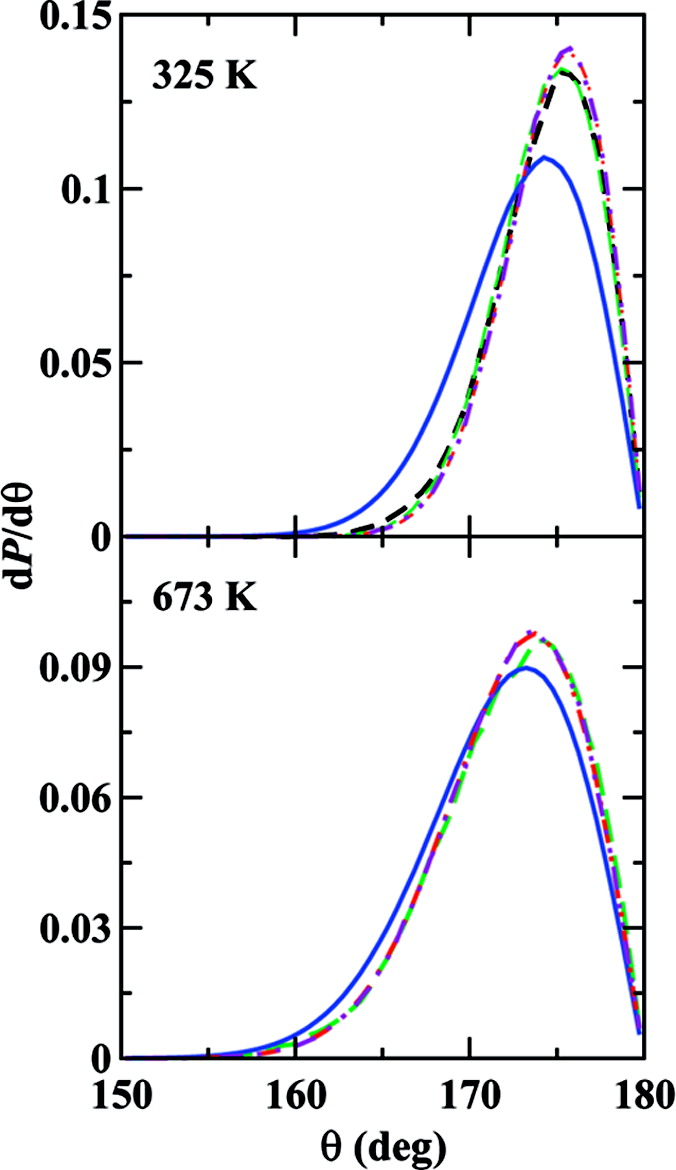
Recent work has focused attention on possible shifts in the bond angle distribution of CO2 as a consequence of intermolecular interactions in the supercritical phase. To investigate the temperature and phase dependence of the intramolecular structure of CO2, we performed Feynman path integral Monte Carlo calculations based on a spectroscopically derived analytical potential, first principles molecular dynamics simulations using Kohn−Sham density functional theory, and Monte Carlo simulations employing empirical interaction potentials. On the basis of various distributions used to characterize the intramolecular structure, we conclude that the aggregation state has a negligible influence on the intramolecular structure, in particular we find that in the classical limit the distributions are remarkably similar for the ideal gas, supercritical, and solid phases when considered at the same temperature. In contrast, an increase in the temperature from 325 to 673 K or inclusion of nuclear quantum effects leads to a significant broadening of the distributions. With respect to the first C−O bond vector, the second bond vector most prefers a collinear arrangement. However, due to the Jacobian factor the maximum in the bond angle distribution at 325 K is shifted to an angle of about 175.7° in the classical limit or to 173.0° if nuclear quantum effects are included. Nevertheless, an analysis of the temperature dependence of the constant-volume heat capacity demonstrates that carbon dioxide should be viewed as a linear molecule.