J. Phys. Chem. B 115, 11431-11438 (2011)
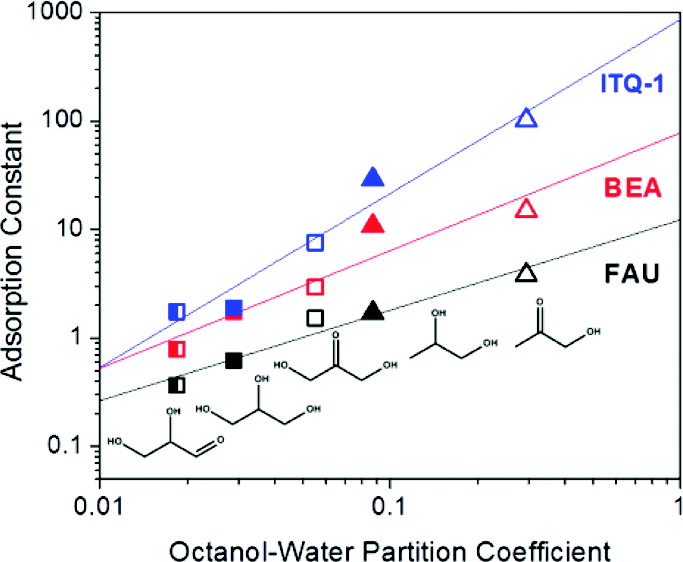
Henry’s constants (Kads) for adsorption of C3 polyfunctional molecules onto zeolites from aqueous solutions at 278 K were obtained and compared with the octanol–water partition coefficients, Kow, which were calculated using the prevalent ClogP group contribution method. Kads increases linearly with Kow for these adsorbates on H–ZSM-5 (MFI), FAU, BEA, and ITQ-1 (MWW). Kads values for C2–C6 diol adsorption at 278 K are also linearly correlated with Kow regardless of interactions in the bulk phase as measured by the solution activity coefficient. Exceptions to the correlation established between Kads and Kow are the adsorption of 1,2,ω-triols with carbon number greater than three on H–ZSM-5 and adsorption of all oxygenates studied on FER, which we postulate to be due to the effect of changing adsorption configuration with adsorbate/zeolite structure which cannot be captured by Kow alone. These results enable the prediction of separation selectivities of biomass-derived compounds on zeolite adsorbents.