J. Chromatogr. A 1218, 9183-9193 (2011)
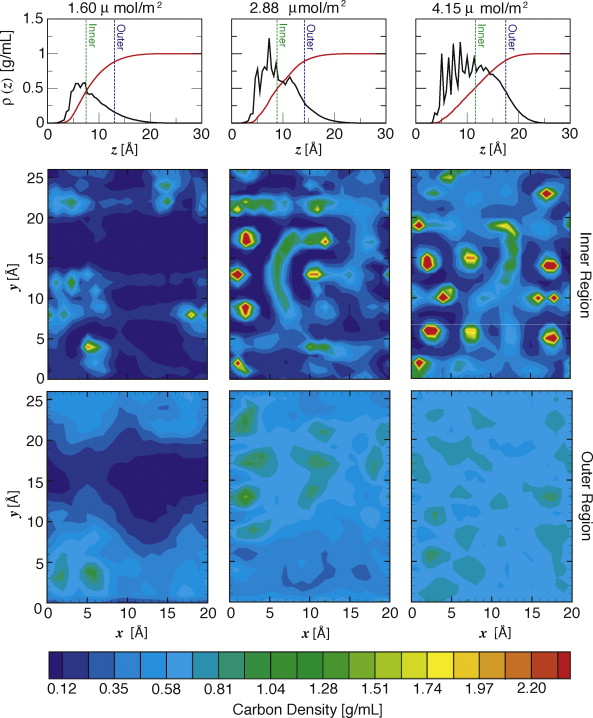
Reversed-phase liquid chromatography (RPLC) is the foremost technique for the separation of analytes that have very similar chemical functionalities, but differ only in their molecular shape. This ability is crucial in the analysis of various mixtures with environmental and biological importance including polycyclic aromatic hydrocarbons (PAHs) and steroids. A large amount of effort has been devoted to studying this phenomenon experimentally, but a detailed molecular-level description remains lacking. To provide some insight on the mechanism of shape selectivity in RPLC, particle-based simulations were carried out for stationary phases and chromatographic parameters that closely mimic those in an experimental study by Sentell and Dorsey [J. Chromatogr. 461 (1989) 193]. The retention of aromatic hydrocarbons ranging in size from benzene to the isomeric PAHs of the formula C18H12 was examined for model RPLC systems consisting of monomeric dimethyl octadecylsilane (ODS) stationary phases with surface coverages ranging from 1.6 to 4.2 μmol/m2 (i.e., stationary phases yielding low to intermediate shape selectivity) in contact with a 67/33 mol% acetonitrile/water mobile phase. The simulations show that the stationary phase acts as a very heterogeneous environment where analytes with different shapes prefer different spatial regions with specific local bonding environments of the ODS chains. However, these favorable retentive regions cannot be described as pre-existing cavities because the chain conformation in these local stationary phase regions adapts to accommodate the analytes.