AIChE J. 59, 3523-3529 (2013)
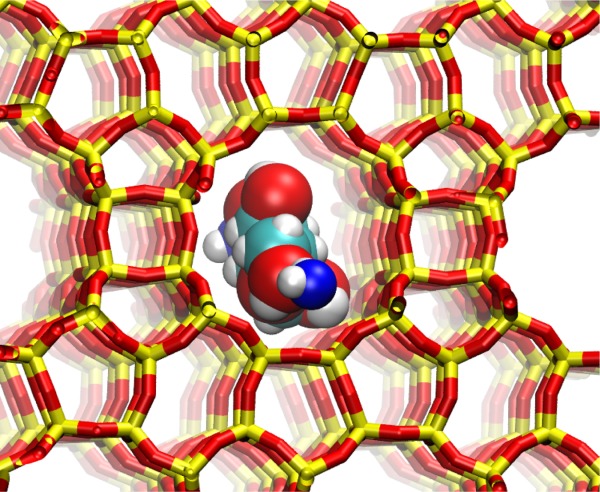
Hydrophobic zeolites, including Ti‐ and Sn‐beta, have been found to adsorb and isomerize glucose into fructose. An experimental question has been the significance of the entropic contribution to the free energy of transfer of glucose from solution to zeolite. We here perform Gibbs ensemble Monte Carlo calculations to quantify the enthalpy, entropy, and free energy of transfer of glucose from the aqueous phase to the zeolite phase. We find that the entropic contribution is large and positive, nearly compensating for an unfavorable enthalpy of transfer in all‐silica zeolite beta. A significant component of the positive entropy of transfer from the aqueous phase to zeolite is the unstructuring of first coordination shell waters around glucose as it leaves the solution.