ChemPhysChem 19, 512-518 (2018)
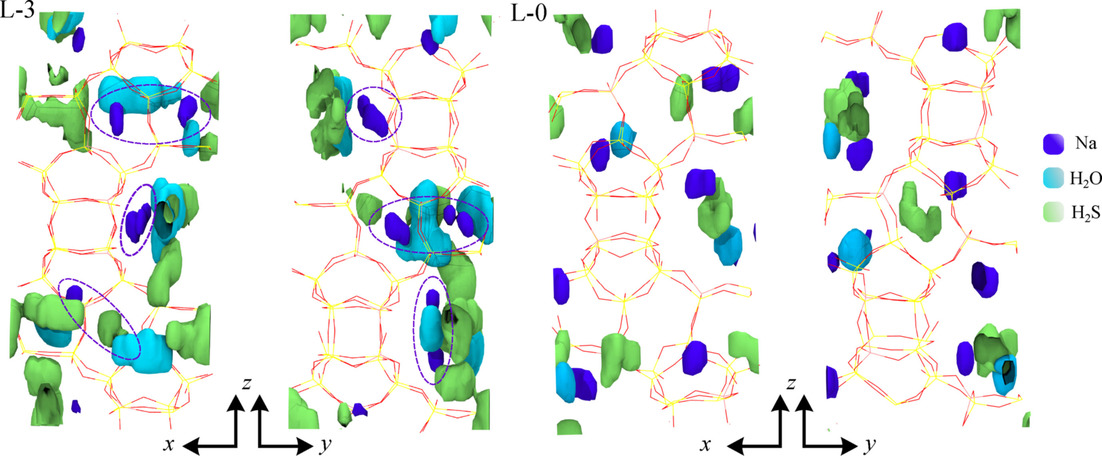
Purifying sour natural gas streams containing hydrogen sulfide and carbon dioxide has been a long‐standing environmental and economic challenge. In the presence of cation‐exchanged zeolites, these two acid gases can react to form carbonyl sulfide and water (H2S+CO2⇌H2O+COS), but this reaction is rarely accounted for. In this work, we carry out reactive first‐principles Monte Carlo (RxFPMC) simulations for mixtures of H2S and CO2 in all‐silica and Na‐exchanged forms of zeolite beta to understand the governing principles driving the enhanced conversion. The RxFPMC simulations show that the presence of Na+ cations can change the equilibrium constant by several orders of magnitude compared to the gas phase or in all‐silica beta. The shift in the reaction equilibrium is caused by very strong interactions of H2O with Na+ that reduce the reaction enthalpy by about 20 kJ mol−1. The simulations also demonstrate that the siting of Al atoms in the framework plays an important role. The RxFPMC method presented here is applicable to any chemical conversion in any confined environment, where strong interactions of guest molecules with the host framework and high activation energies limit the use of other computational approaches to study reaction equilibria.