J. Phys. Chem. B 104, 2415-2423 (2000)
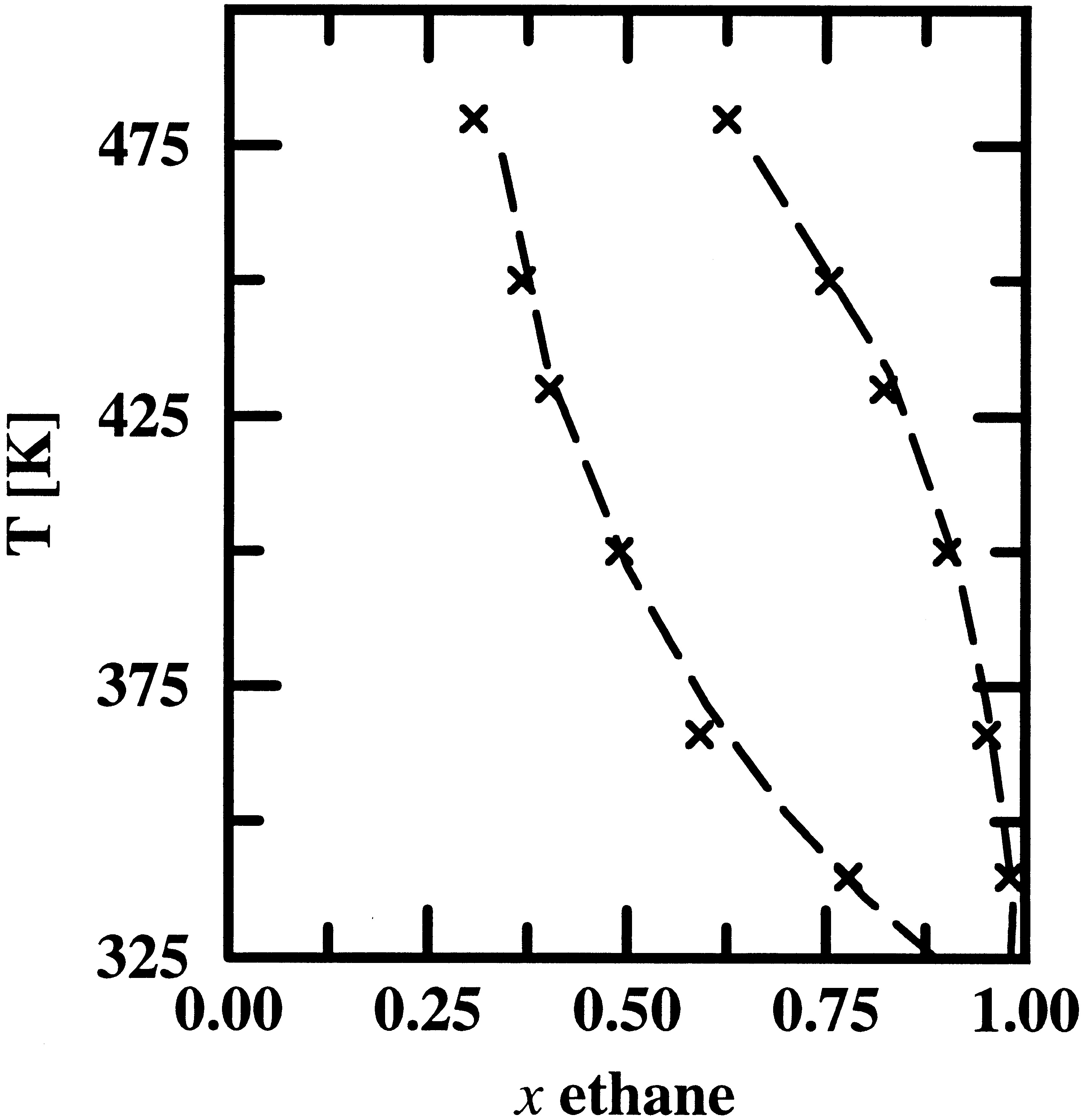
Pressure-composition and temperature-composition phase diagrams are computed for the binary mixture of n-heptane and supercritical ethane using four different molecular models of increasing complexity. The ethane and heptane molecules were described using either (i) single-site Lennard−Jonesium (LJ) with fixed well-depth and size parameters, (ii) single-site LJ with temperature-dependent well depths, (iii) chains of methyl and methylene pseudo-atoms interacting via LJ potentials placed at the positions of all carbon nuclei, or (iv) an explicit-hydrogen representation with LJ interaction sites placed both at the carbon nuclei and at the centers of all carbon−hydrogen bonds. For comparison, simulations were also performed for the binary mixture of n-heptane and helium using the pseudo-atom model for the n-heptane molecules. All four models produce phase diagrams for the ethane/n-heptane mixture that are in reasonable agreement with experiment. However, the accuracy of the calculated phase diagrams improves markedly with increasing complexity of the model (and therefore increasing computational requirements). In both the liquid (n-heptane-rich, higher specific density) and supercritical (ethane-rich, lower specific density) phases center-of-mass radial distribution functions appear to show more enhanced structures for the two single-site models than the united-atom and explicit-hydrogen force fields. However, the number integrals of these radial distribution functions are strikingly similar for all models, that is the differences in molecular shape do not lead to a difference in the clustering of the solvent molecules around the solute. In particular, preferential solvation of n-heptane by ethane is not observed in the supercritical phase. Analysis of the contributions of the liquid and the supercritical phases to the decrease of the Gibbs free energy of transfer of n-heptane with increasing pressure suggest that the enhanced solubility of n-heptane in high-pressure supercritical ethane can be attributed to two causes of roughly equal importance: “Pulling” of n-heptane into the supercritical phase by an increased density of ethane that acts as a nonspecific solvent, and “pushing” n-heptane out of the liquid phase by an increased concentration of ethane.