J. Phys. Chem. B 104, 8008-8016 (2000)
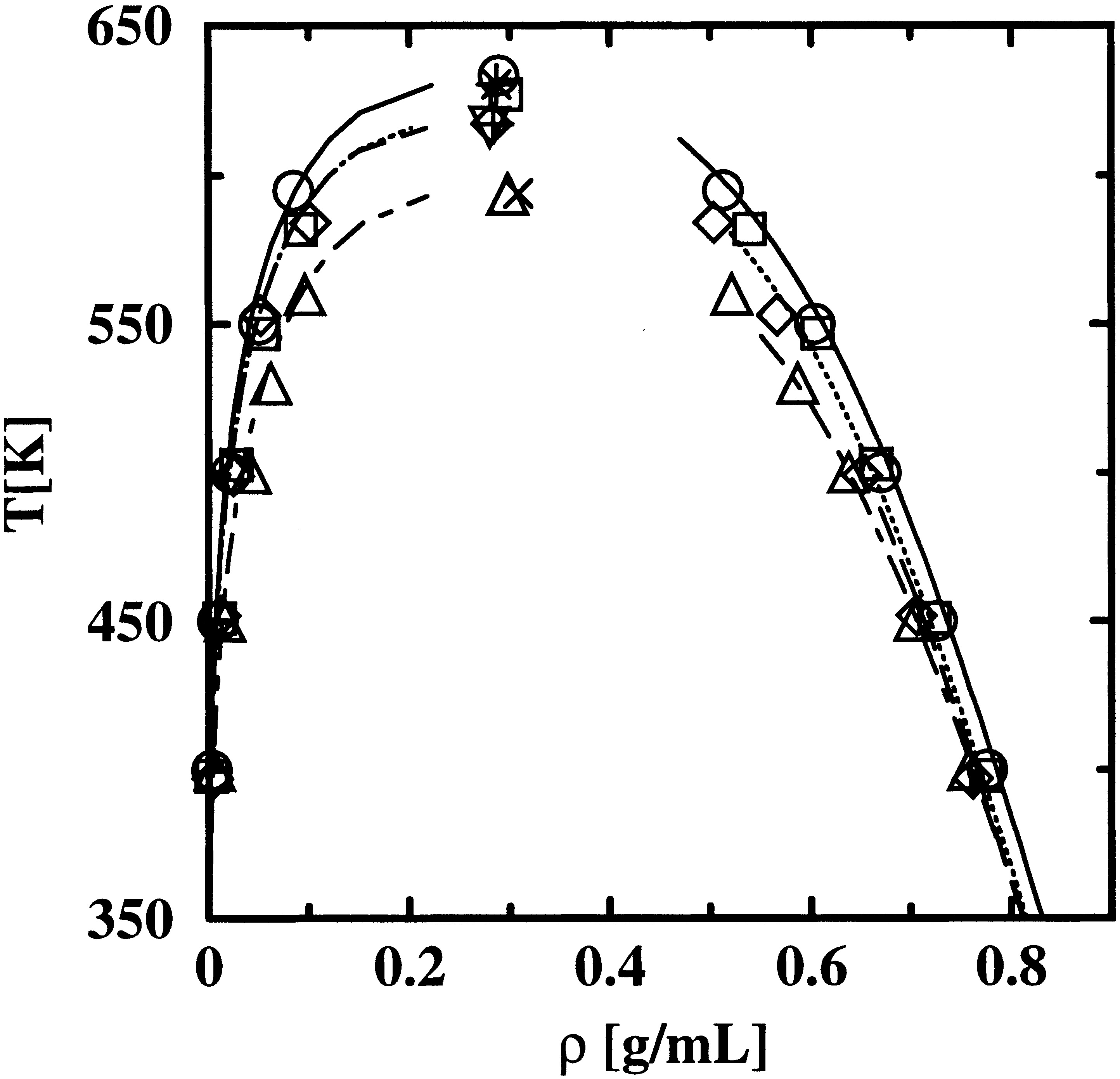
The Transferable Potentials for Phase Equilibria-United Atom (TraPPE-UA) force field for hydrocarbons is extended to alkenes and alkylbenzenes by introducing the following pseudo-atoms: CH2(sp2), CH(sp2), C(sp2), CH(aro), R−C(aro) for the link to aliphatic side chains and C(aro) for the link of two benzene rings. In this united-atom force field, the nonbonded interactions of the hydrocarbon pseudo-atoms are solely governed by Lennard-Jones 12−6 potentials, and the Lennard-Jones well depth and size parameters for the new pseudo-atoms were determined by fitting to the single-component vapor−liquid-phase equilibria of a few selected model compounds. Configurational-bias Monte Carlo simulations in the NVT version of the Gibbs ensemble were carried out to calculate the single-component vapor−liquid coexistence curves for ethene, propene, 1-butene, trans- and cis-2-butene, 2-methylpropene, 1,5-hexadiene, 1-octene, benzene, toluene, ethylbenzene, propylbenzene, isopropylbenzene, o-, m-, and p-xylene, and naphthalene. The phase diagrams for the binary mixtures of (supercritical) ethene/n-heptane and benzene/n-pentane were determined from simulations in the NpT Gibbs ensemble. Although the TraPPE-UA force field is rather simple and makes use of relatively few different pseudo-atoms, its performance, as judged by comparisons to other popular force fields and available experimental data, is very satisfactory.