Anal. Chem. 74, 37-44 (2002)
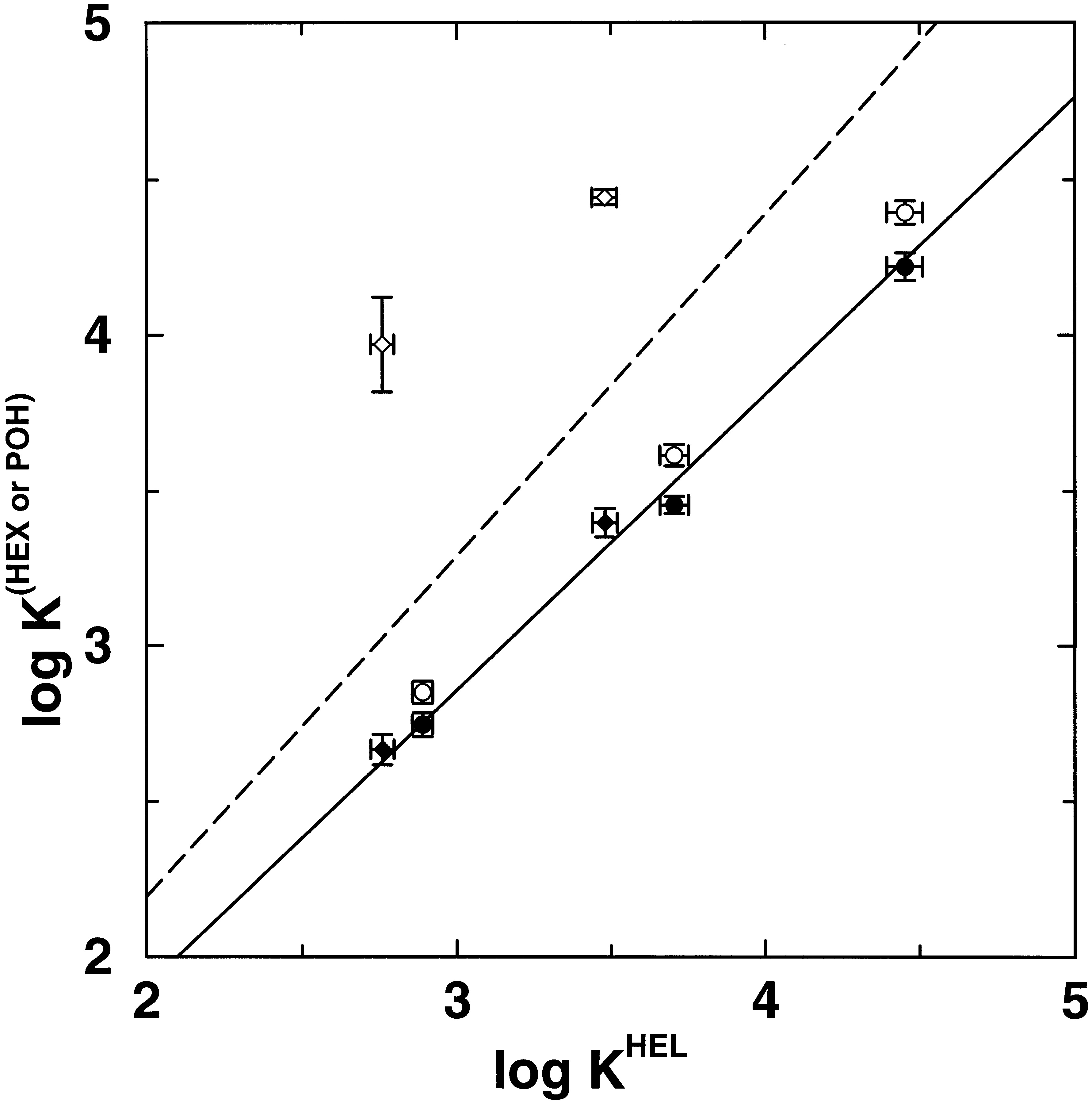
In an attempt to elucidate the molecular basis for concentration (isotherm) effects on retention in gas−liquid chromatography, configurational-bias Monte Carlo simulations in the Gibbs ensemble were carried out to investigate changes in analyte partitioning caused by overloading a model chromatographic system with either an alkane or an alcohol. Squalane was used as the stationary-phase material, and the analytes included n-pentane, n-hexane, n-heptane, 1-butanol, and 1-pentanol. Three systems were studied that differed in the mobile-phase composition: (i) a helium vapor, (ii) a n-hexane vapor, and (iii) a 1-pentanol-saturated helium vapor. While the amount of helium that partitions into the stationary phase is very small, both n-hexane and 1-pentanol partition strongly into and thereby swell the stationary phase. Although the swelling of the stationary phase leads to a reduction in the partition coefficients for the alkane solutes for both the n-hexane- and 1-pentanol-swollen stationary phases, the effects on the alcohol solutes differ markedly. Whereas saturation by n-hexane causes a decrease of the alcohol partition contants (to an extent similar to that for the alkane solutes), the saturation by 1-pentanol causes a dramatic increase of the alcohol partition coefficients; e.g., the Kovats index of 1-butanol increases by more than 150 Kovats units. The formation of hydrogen-bonded alcohol aggregates in the liquid phase is the microscopic origin for the dramatic effect of 1-pentanol saturation on the retention of alcohols.