J. Phys. Chem. B 107, 12320-12323 (2003)
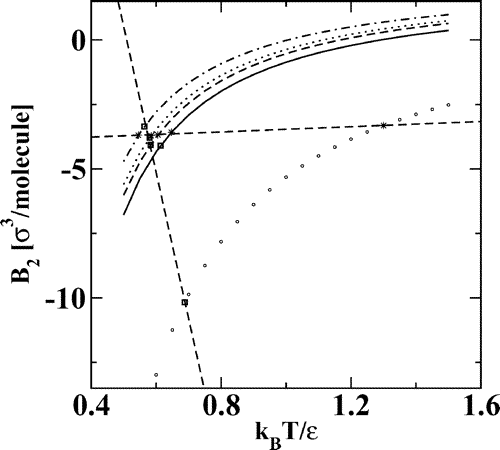
Gibbs ensemble Monte Carlo simulations were carried out to calculate the vapor−liquid and vapor−solid coexistence curves for three fullerenes: C60, C70, and C96. Single-site potentials with parameters proposed by Girifalco and Pacheco/Ramalho were used to describe the interactions of the fullerene molecules. It is observed that the liquid-phase temperature range (as measured by the reduced triple point temperature, Tt/Tc) decreases considerably for C60 compared to 12−6 Lennard-Jonesium and eventually disappears for C96 as the potential wells become narrower with increasing molecular weight of the fullerenes. This confirms previous theoretical predictions that the width of the potential well has an important influence on the overall shape of a phase diagram. However, calculations of reduced second virial coefficients show that the fullerene phase diagrams can be described by an extended principle of corresponding states. The Gibbs ensemble simulations yield a triple point temperature of 1876 ± 13 K for C60 and the Girifalco potential, in excellent agreement with recent calculations by Hasegawa and Ohno and Costa et al.