J. Phys. Chem. B 108, 12990-12998 (2004)
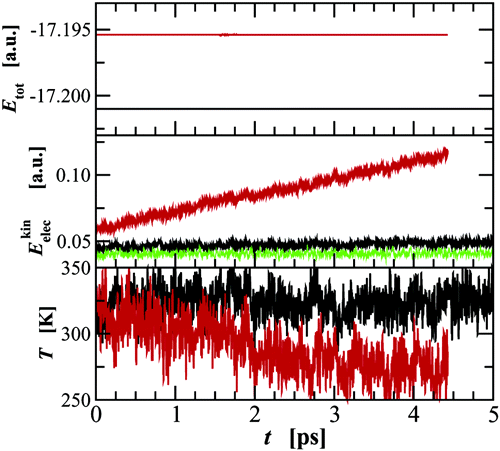
A series of first principles molecular dynamics and Monte Carlo simulations were carried out for liquid water to investigate the reproducibility of different sampling approaches. These simulations include Car−Parrinello molecular dynamics simulations using the program cpmd with different values of the fictitious electron mass in the microcanonical and canonical ensembles, Born−Oppenheimer molecular dynamics using the programs cpmd and cp2k in the microcanonical ensemble, and Metropolis Monte Carlo using cp2k in the canonical ensemble. With the exception of one simulation for 128 water molecules, all other simulations were carried out for systems consisting of 64 molecules. Although the simulations yield somewhat fortuitous agreement in structural properties, analysis of other properties demonstrate that one should exercise caution when assuming the reproducibility of Car−Parrinello and Born−Oppenheimer molecular dynamics simulations for small system sizes in the microcanonical ensemble. In contrast, the molecular dynamics and Monte Carlo simulations in the canonical ensemble appear to be more reliable. Furthermore, in the case of canonical Car−Parrinello molecular dynamics simulations the application of Nosé−Hoover chain thermostats allows the use of larger fictitious electron masses. For the Becke−Lee−Yang−Parr exchange and correlation energy functionals and norm-conserving Troullier−Martins or Goedecker−Teter−Hutter pseudopotentials, these simulations at a fixed density of 1.0 g/cm3 and a temperature close to 315 K point to an overstructured liquid with a height of the first peak in the oxygen−oxygen radial distribution function of about 3.0, an underestimated value of the classical constant-volume heat capacity of about 70 J/(mol K), and an underestimated self-diffusion constant of about 0.04 Å2/ps.