J. Phys. Chem. B 108, 17596-17605 (2004)
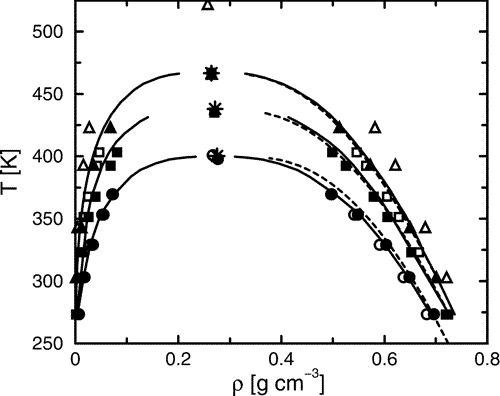
The extension of the transferable potentials for phase equilibria−united atom (TraPPE−UA) force field to the ether, glycol, ketone, and aldehyde functionalities is presented. New parameters for the ether oxygen, the carbonyl carbon (ketones), the carbonyl methine (aldehydes), and a special intramolecular hydrogen-bond term were fitted to the vapor−liquid coexistence curves for selected one-component systems. Coupled−decoupled configurational bias Monte Carlo simulations in the Gibbs or grand canonical ensemble were used to compute the vapor−liquid coexistence curves for the neat systems of dimethyl ether, ethyl methyl ether, diethyl ether, dipropyl ether, diisopropyl ether, methyl tert-butyl ether, 1,2-ethanediol, 2-methoxyethan-1-ol, 1,2-dimethoxyethane, 1,3-propanediol, acetone, 2-pentanone, 2-octanone, acetaldehyde, pentanal, and octanal. Additional simulations were performed for the binary mixtures of diethyl ether + ethanol and acetone + hexane. Excellent agreement with experimental results was found with the mean unsigned errors being less than 1% for the critical temperatures and about 3% (ethers) and 1% (other) for the normal boiling temperatures. For the mixture of acetone + hexane at 328.15 K, a positive pressure azeotrope was found with xacetoneazeo = 0.71 in satisfactory agreement with the experimental result of 0.64. Additionally, the structures of hydrogen-bonded aggregates were investigated for 1,2-ethanediol and 2-methoxyethan-1-ol, where the average hydrogen-bond energies were found to be about −20 and −14 kJ mol-1 for inter- and intramolecular hydrogen bonds, respectively.