J. Chromatogr. A 1079, 127-135 (2005)
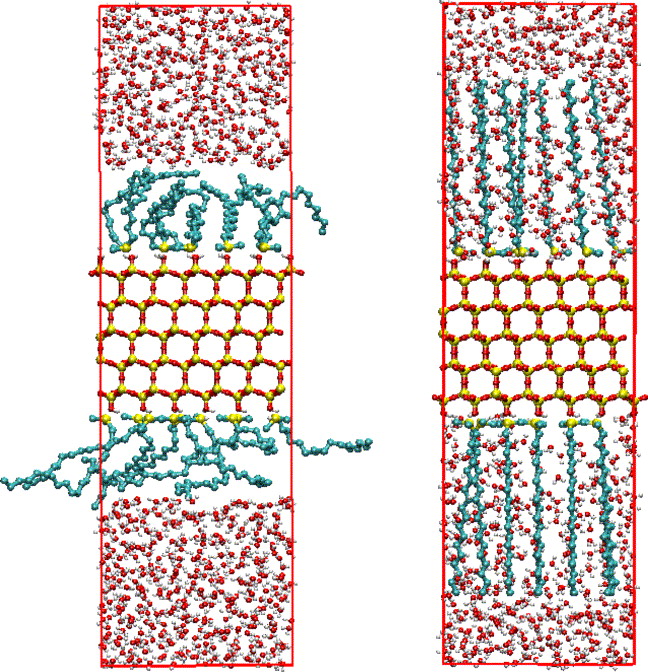
The dramatic loss of retention in reversed-phase liquid chromatography when switching to 100% aqueous solvent and stopping flow (depressurizing) has long intrigued separation scientists. Recent experimental evidence suggests that the observed loss of retention is due to the loss of pore wetting with subsequent loss of solvent penetration in the porous matrix. One of the prevalent explanations of this phenomenon has been that the bonded phase chains, typically octadecyl silane bound to porous silica, would undergo significant conformational changes, viz. collapse, under pure aqueous conditions. As a definitive means toward elucidating the conformation of bonded-phase chains under pure aqueous conditions, configurational-bias Monte Carlo simulations in the Gibbs ensemble were carried out for a system of dimethyl octadecyl silane of intermediate coverage bound to the (1 1 1) face of β-cristobalite and immersed in pure water. The results of two sets of simulations, which were started from two entirely different starting configurations as a validity check toward reaching the same equilibrium distribution of states, show that chains are neither clustering together nor laying on the surface but rather have a broad distribution of orientations and of conformational states. The interface between the bonded and solvent phases is rough on a molecular level, and clusters of water molecules are sometimes found to adsorb at the silica surface. This computational study lends further evidence that the driving force for the loss of retention when switching to pure aqueous conditions and depressurizing is not the collapse of bonded-phase chains.