Theor. Chem. Acc. 115, 391-397 (2006)
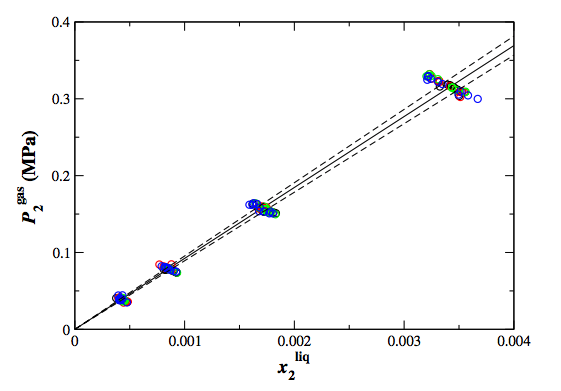
Configurational-bias Monte Carlo simulations in the Gibbs ensemble were used to calculate Henry’s law constants, Ostwald solubilities, and Gibbs free energies of transfer for oxygen, nitrogen, methane, and carbon dioxide in ethanol at 323 and 373 K. These three solubility descriptors can be expressed as functions of mechanical properties that are directly observable in the Gibbs ensemble approach, thereby allowing for very precise determination of the descriptors. Additionally, the Henry’s law constants of multiple solutes can be computed from a single simulation. Most of the simulations were carried out for systems containing 1,000 solvent and up to 8 solute molecules, and further simulations using either 500 or 2,000 solvent molecules point to negligible system size effects. A comparison with experimental data shows that the united-atom version of the transferable potential for phase equilibria force field yields Henry’s law constants that reproduce well the differences between the four solutes and the changes upon increase of the temperature.