J. Phys. Chem. B 110, 10519-10525 (2006)
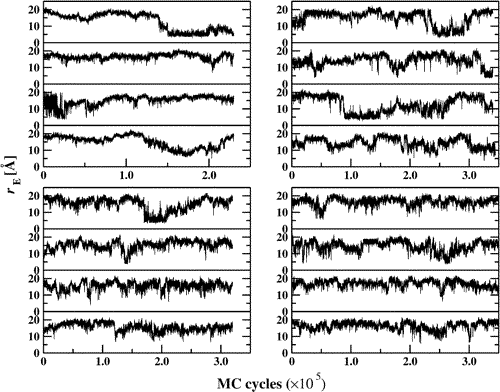
Configurational-bias Monte Carlo simulations in the isobaric−isothermal ensemble (T = 323 K and p = 10 atm) were carried out to probe structural properties of an isolated n-octadecane chain solvated in water, methanol, water-rich, or methanol-rich mixtures and, for comparison, of an isolated chain in the gas phase and for neat liquid n-octadecane. The united-atom version of the TraPPE (transferable potentials for phase equilibria) force field was used to represent n-octadecane and methanol and the TIP-4P model was used for water. In all six environments, broad conformational distributions are observed and the n-octadecane chains are found to predominantly adopt extended, but not all-trans conformations. In addition, a small fraction of more collapsed conformations in which the chain ends approach each other is observed for aqueous hydration, the water-rich solvent mixture and the gas phase, but the simulation data do not support a simple two-state picture with folded and unfolded basins of attraction. For chains in these three “poor” solvent environments, the dihedral angles near the center of the chain show an enhancement of the gauche population. The ensemble of water-solvated chains with end-to-end contacts is preferentially found in a U-shaped conformation rather than a more globular state. An analysis of the local solvation structures in the water−methanol mixtures shows, as expected, an enrichment of the methyl group of methanol near the methylene and methyl segments of the n-octadecane chain. Interestingly, these local bead fractions are enhanced by factors of 2.5 and 1.5 for methyl and methylene segments reflecting the more hydrophobic nature of the former segments.