Fluid Phase Equil. 183-184, 301-309 (2001)
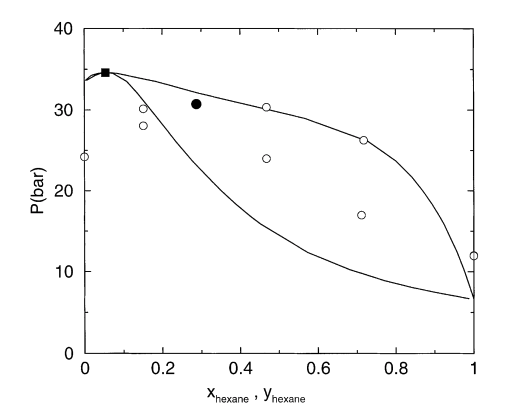
Configurational-bias Monte Carlo simulations were carried out to investigate (i) the self-aggregation of 1-hexanol in n-hexane rich binary solutions; (ii) the partitioning of normal alkane and primary alkanol solutes between water, neat or water-saturated 1-octanol, n-octane, and vapour phases, and (iii) the vapour–liquid phase diagram of the binary mixture of methanol and n-hexane at a temperature of 448 K. The optimized potentials for liquid simulations (OPLS) united-atom force field was used to describe the interactions of the alkanes and alcohols, and the transferable intermolecular potentials 4 point (TIP4P) model was used for water. Analysis of radial distribution functions and their corresponding number integrals supports a micro-heterogenous picture of the 1-hexanol/hexane solutions with the majority of the 1-hexanol molecules being part of hydrogen-bonded clusters with four to six molecules. The OPLS force field yields Gibbs free energies of transfer that are in qualitative, albeit not quantitative agreement with experimental results. Comparison of the partitioning between a helium vapour phase and dry and wet (water-saturated) 1-octanol established that water saturation affects mostly the partitioning of polar solutes, while differences for alkane partitioning were found to be negligible. The vapour pressure of OPLS n-hexane is too low and that of methanol too high, resulting in a binary vapour–liquid phase diagram that deviates significantly from experiment.