Macromolecules 54, 1120–1136 (2021)
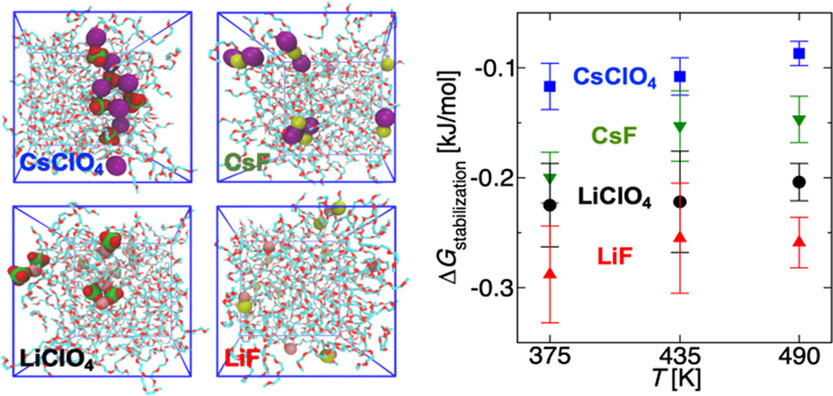
Gibbs ensemble Monte Carlo simulations for salt-doped oligo(ethylene oxide) (OEO, Mw = 90–266 g/mol) solutions show that the presence of ions leads to significant increases in the cohesive energy density (ΠCED) and the enthalpy of vaporization for OEO chains but that compensation by entropic contributions leads to only small changes in the Gibbs free energy of transfer and vapor pressure. At the same relative ion concentration (r) and temperature, the ΠCED values of the salt-doped systems order as LiClO4 > LiF > CsClO4 ≈ CsF. Structural analysis indicates significant ion clustering in addition to coordination of cations by OEO chains. After accounting for ion clustering via the van’t Hoff factor, the solvent vapor pressures are well described by Raoult’s law. Experiments and simulations for a squalane/tetraethylene glycol dimethyl ether blend (xW,OEO = 0.65) show that the addition of LiClO4 does not significantly alter the miscibility gap below 0.95 TCP,free, the critical temperature of the salt-free blend. However, the coexistence curve for the LiClO4-doped system does not close with the usual power-law scaling at T > 0.95 TCP,free as transfer of OEO chains to the squalane-rich phase leads to an increase in r in the OEO-rich phase, which, in turn, makes it a less hospitable environment for squalane.